Iron isotopic composition and atomic weight of selected iron-bearing... | Download Scientific Diagram

1 Atomic Number The atomic number is equal to the number of protons in the nucleus. Sometimes given the symbol Z. On the periodic chart Z is the uppermost. - ppt download

Atomic weight of an element X is 120 when one amu is defined as 1//18th part by weight of an ele... - YouTube

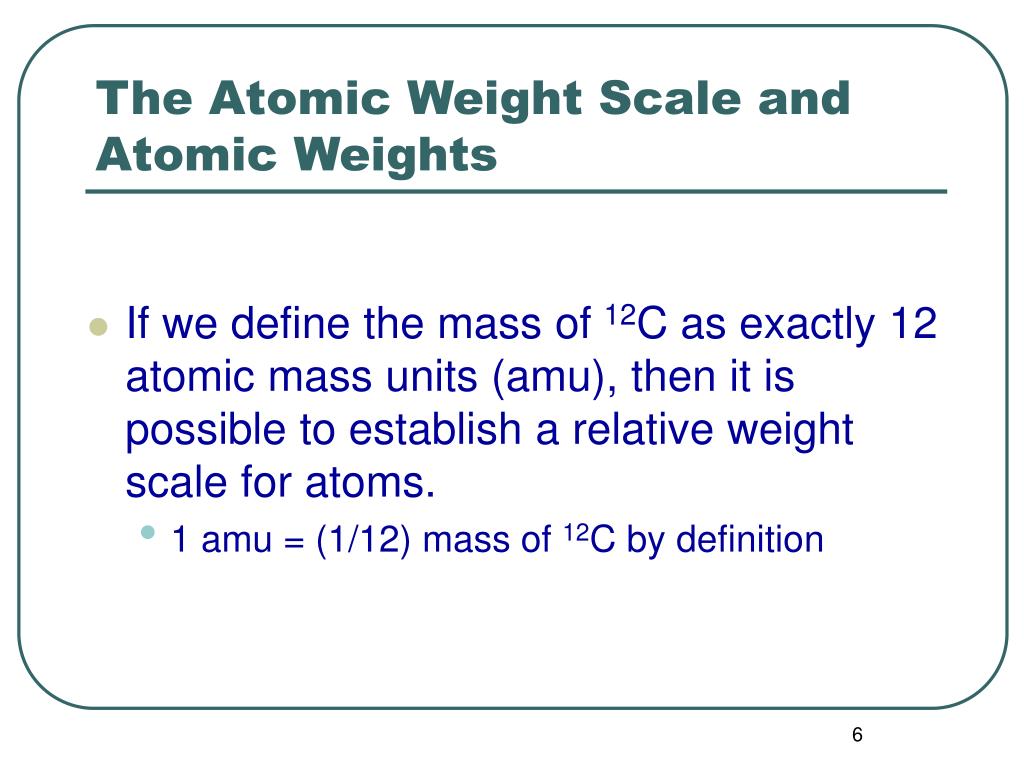
5 no The modern atomic weight scale is based on (A) 016 Maximum number of atoms are present in - Chemistry - Some Basic Concepts of Chemistry - 11528169 | Meritnation.com



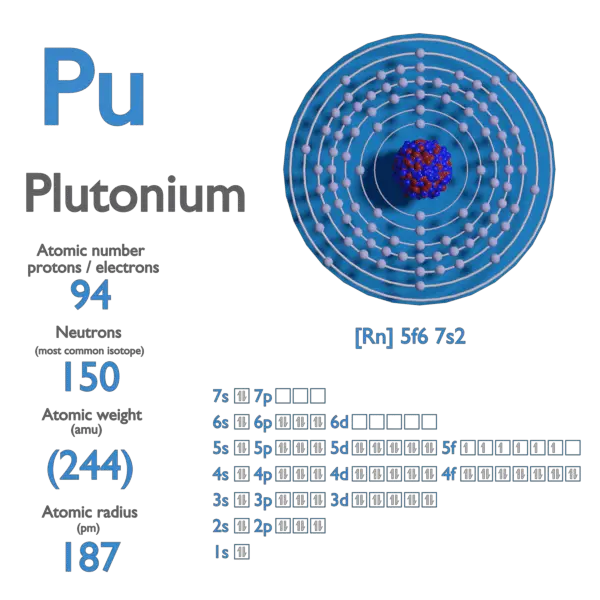


:max_bytes(150000):strip_icc()/boron-illustration-545864379-5838819f5f9b58d5b1c57b5f.jpg)